Structure of the atom class 9 notes
Structure of the atom class 9 notes are very important for those students who love chemistry (not with a girl) from ncert book
So in this article we let you take a look to structure of the atom class 9 notes and some extra questions and provide you a beautiful pdf in the last.
Why structure of the atom class 9 chapter made? And why we are reading structure of the atom class 9 notes?
Atoms and molecules are fundamental building blocks of matter
In previous chapter we have read that atoms are indivisible but many questions were there contradictory
When we rub two materials they attain a charge . Where does this charge come from?
How does current flow in a wire what is actually moving ?
After that a series of experiment revealed that atoms consisted of some more smaller particles called subatomic particles ie electrons protons neutrons.
Structure of the atom class 9 notes
What we are going to read in the structure of the atom class 9 notes?
- Charged particles in Matter
- Structure of an atom
- Writing chemical formulae
- Atomic and Mass number
- Different Atomic species
Structure of the atom class 9 notes (Charged particles in Matter)
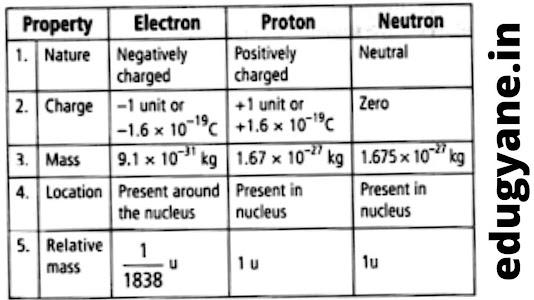
ELECTRONS
Discovered by J.J. Thompson.
Negatively charged particle denoted by ‘e-’
Contains a charge of -1.6 X 10-16
He performed a cathode ray experiment using a discharge tube in which he observed cathode rays.
CATHODE RAYS - Cathode rays are negatively charged rays which are seen moving from the cathode towards the anode in a specially designed discharge tube , when a high potential difference is applied across the electrodes under very low pressure.
PROTONS
Discovered by E.Goldstein in 1886
He also did the same experiment but the cathode was porous this time (since the protons were larger in size as compared to the electrons ) and observed anode rays or canal rays flowing in the opposite direction of cathode rays.
ANODE RAYS OR CANAL RAYS - Canal rays or anode rays are the positively charged rays which are seen moving from the anode towards cathode in specially designed discharge tubes when a high potential difference is applied among the electrodes.
Positively charged particles
Denoted by ‘p+’
Contains a charge of +1.6 X 10-16
The mass of proton is approximately 2000 times that of electron.(perfect value is 1840)
NEUTRONS
CONCLUSION
The mass of proton is taken as 1 unit and its charge is taken as +1
The mass of electrons is taken as negligible and its charge is taken as -1 unit.
STRUCTURE OF AN ATOM (structure of the atom class 9 notes)
(IMPORTANT TOPIC)
How are these subatomic particles placed in an atom?
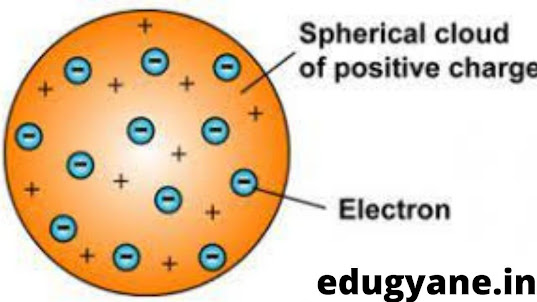
THOMSON'S MODEL OF AN ATOM (PLUM PUDDING MODEL)
J.J.Thompson was the first one to propose a model of structure of an atom
He said that the positive charge is uniformly distributed in an atom and negative particles are embedded in it
Just like a pudding or a watermelon (red-edible part is positive charge and the seeds are negatively charged particles)
POSTULATES
Following are the postulates of this model:-
- Electrons are embedded in the sphere of positive charge.
- The negative and positive charges are equal in magnitude.Therefore, the atom as a whole is electrically neutral.
- The mass of an atom is assumed to be uniformly distributed throughout the atom.
Limitations of Thomson's Model of an Atom
- J.J. Thomson's model could not explain the experimental results of other scientists such as Rutherford, as there is no nucleus in the atomic model proposed by Thomson.
- It could not explain the stability of an atom, i.e. how positive and negative charges could remain so close together.
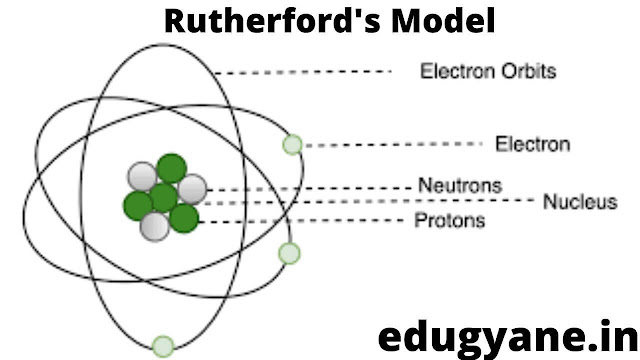
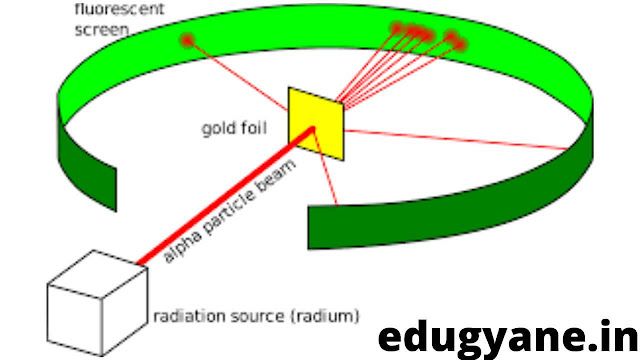
Ernest Rutherford designed an experiment to know how the electrons are arranged within an atom.
EXPERIMENT
He bombarded thin sheets of gold foil with fast moving α-particles (these are doubly charged helium ions having a mass of 4 u).
GOLD FOIL HI KYU? JYADA AMEER THA KYA? 😆
He selected a gold foil because he wanted a layer as thin as possible. This gold foil was about 1000 atoms thick.
OBSERVATIONS
- Most of the fast moving α-particles passed straight through the gold foil.
- Some of the a-particles were deflected by the foil by small angles.
- Very few a-particles (one out of 12000) appeared to rebound.
- Most of the space inside the atom is empty because most of the α-particles passed through the gold foil without getting deflected.
- Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
- A very small fraction of α-particles deflected by 180° (they rebounded) indicating that all the mass and the positive charge of the atom were concentrated in a very small volume within the atom.
POSTULATES
- There is a positively charged, highly dense centre in an atom, called the nucleus. Nearly, the whole mass of the atom resides in the nucleus.
- The electrons revolve around the nucleus in a circular path.
- The size of the nucleus (10-15 m) is very small as compared to the size of the atom (10-10m).{1 LAKH TIMES SMALLER}
- Any charged particle when acceleration is expected to radiate energy. To remain in a circular orbit, the electron would need to undergo acceleration. Therefore, it would radiate energy. Thus, the revolving electron would lose energy and finally fall into the nucleus.If this were so, the atom should be highly unstable. Therefore, matter would not exist, but we know matter exists. It means that atoms are quite stable. Thus, it could not explain the stability of an atom when charged electrons are moving under the attractive force of a positively charged nucleus.
- Rutherford's model could not explain the distribution of electrons in the extra nuclear portion of the atom{nucleus ke alava bacha hua area}
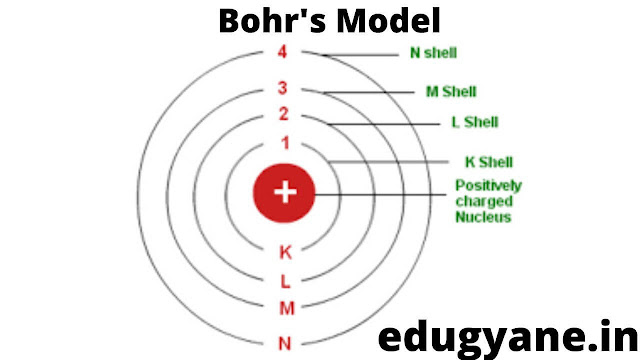
- Atom consists of a positively charged nucleus around which electrons revolve in discrete orbits i.e. electrons revolve in permissible orbits and not just in any orbit.
- Starting from the nucleus, energy levels (orbits) are represented by numbers (1, 2, 3, 4 etc.) or by alphabets (K, L, M, N etc.).
- Energy of an electron remains the same as long as it remains in discrete orbit and it does not radiate energy while revolving.
- The maximum number of electrons present in a shell is given by the formula 2n², where n is the orbit number or energy level 1, 2, 3,....
- Therefore, the maximum number of electrons in different shells are as follows:
- First orbit or K-shell = 2 × (1)² = 2
- Second orbit or L-shell = 2 × (2)² = 8
- Third orbit or M-shell = 2 x (3)² = 18
- Fourth orbit or N-shell = 2 × (4)² = 32 and so on.
- The maximum number of electrons that can be accommodated in the outermost orbit is 8.
- Electrons are not accommodated in a given shell, unless the inner shells are filled (i.e. the shells are filled in a stepwise manner).
Structure of the atom class 9 notes (Atomic and Mass number)
ATOMIC NUMBER
No. of protons = the atomic number of the element
All the atoms of the same element have sae number of protons and hence same atomic number
Denoted by ‘Z’
p=Z
MASS NUMBER
The sum of the number of protons and the number of protons (collectively known as nucleons) is known as mass number.
no. of protons + no. of neutrons = mass number
Denoted by ‘A’
A=p+n
Structure of the atom class 9 notes (Different Atomic Species)
- An isotope of uranium (U-235) is used as a fuel for the production of electricity in nuclear reactors.
- U-238 is used to determine the age of very old rocks and even the age of the earth.
- An isotope of cobalt (Co-60) is used in the treatment of cancer.
- An isotope of carbon (C-14) is used to determine the age of old specimens of wood or old bones of living organisms.
- An isotope of iodine (1-131) is used in the treatment of goitre.
YOU MIGHT ALSO LIKE
Opinion
Structure of the atom class 9 extra questions
If you have done reading the structure of the atom class 9 notes you have to do some practice from structure of the atom class 9 extra questions and get ready for the unit tests or the upcoming term 2 examinations.
The structure of the atom class 9 extra questions that we are providing you are important because it helps you to practice and test your knowledge about the chapter.
What we are going to read in the structure of the atom class 9 extra questions
- MCQ type Questions
- Very short answer type Questions
- Short answer type Questions
- Long answer time Questions
structure of the atom class 9 extra questions (MCQ type Questions)
1. The first model of an atom was given by
(a) Neil's Bohr
(b) E. Goldstein
(c) Rutherford
(d) JJ Thompson
Structure of the atom class 9 extra questions (solutions available in pdf format at the last)
2. Rutherford's alpha (a) Particles scattering experiment resulted into discovery of
(a) electron
(b) proton
(c) nucleus in the atom
(d) atomic mass
3. Who discovered neutron?
(a) J. Chadwick
(b) Dalton
(c) Bohr
(d) Rutherford
4. The electron distribution in an aluminium atom is
(a) 2,8,3
(b) 2,8,2
(c) 8,2,3
(d) 2,3,8
5. Which one of the following elements has 2,8,8,2 electronic configuration?
(a) Calcium
(b) Copper
(c) Silver
(d) Palladium
6. Elements with Valency 1 are
(a) always metal
(b) always metalloids
(c) either metals or non-metals
(d) always non-metals
7. Isotopes of an element have
(a) the same physical properties
(b) different chemical properties
(c) different numbers of neutrons
(d) different Atomic numbers
8. Which of the following is the valency of an element? If it has 2,8,2 electronic configuration?
(a) 2
(b) 4
(c) 6
(d) 0
9. Which element have 3 atomic numer?
(a) Hydrogen
(b) Carbon
(c) Hilium
(d) Lithium
10. Thompson model of an atom was similar to
(a) Apple
(b) Christmas Pudding
(c) Guava
(d) Zinc
11. What is the electronic configuration of (O)?
(a) 4,4
(b) 2,4,2
(c) 2,8,1
(d) 2,6
12. How many neutrons are there in berellium
(a) 0
(b) 2
(c) 4
(d) 5
13. Which element has the following configuration?
K = 2, L = 8, M = 5
(a) Phosphorus
(b) Nitrogen
(c) Oxygen
(d) Carbon
14. What is the total number of electrons in an atom if first four shells are completely filled?
(a) 36
(b) 32
(c) 26
(d) 48
15. Which of the following have some valency
A(2,8,2), B(2,8,4), C(2,6), D(2,8)?
(a) A and B
(b) B and C
(c) A and C
(d) C and D
16. Which of the following correctly represent the electronic distribution in the Mg atom?
(a) 3,8,1
(b) 2,8,2
(c) 1,8,3
(d) 8,2,2
17. An atom with 3 protons and 4 neutrons will have a valency of
(a) 3
(b) 7
(c) 1
(d) 4
Structure of the atom class 9 extra questions (Very short answer type Questions)
1. What are canal rays?
Structure of the atom class 9 extra questions (solutions available in pdf format at the last)
2. What are anode rays?
3. How many a-particles be represented?
4. Give the number of neutrons in an atom of the element Ag 107/47
5. Does the nucleus contain neutrons also?
6. Why is electron known as universal particle?
7. List two difference between electron, proton and neutron.
8. Express the combining capacity (valency) of Ar40/18
9. How does representation of an atom of an element take place?
10. If an atom contains one electron and one proton, will it carry any charge or not?
11. Compare all the proposed models of an atom give in the chapter.
12. Compare the properties of electrons, protons and neutrons.
13. An element has Z = I1, What is the valency of the element? Also name it?
14. Which of the two elements would be more reactive, element A of atomic number = 36 or element B of atomic number = 19?
15. Who is JJ Thompson?
Structure of the atom class 9 extra questions (short answer type questions)
1. Describe the essential properties of the atomic nucleus. Compare these with the properties of electron.
Structure of the atom class 9 extra questions (solutions available in pdf format at the last)
2. The electronic configurations of some elements are given below. Name the elements.
(i) 2,8,5 (ii) 2,8,8,2 (iii) 2,8,1
3. Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.
4. In response to a question, a student stated that in an atom, the number of protons is greater than the number of neutrons, Which in turn is greater than the number of electrons. Do you agree with the statement? Justify your answer.
5. An atom X has 4 protons and 5 neutrons with electronic configuration 2,2.
(i) atomic number
(ii) mass number
(iii) Valency
6. If an atom of an element has atomic number = 15 and mass number = 31, find the number of protons, electrons, neutrons in its atoms.
7. An element X has 5 electrons in its M-shell. What is it's atomic number?
8. Define valency by taking example of Silicon and Oxygen.
9. How will you, find the valency of chlorine, Sulphur and Magnesium?
10. Why do isotopes of an element have different physical properties?
11. How are the following pairs of atoms related?
8X16, 8X17, 8Y40, 20Z40
12. Write the electronic configuration and valency of the following
(i) chlorine (ii) sodium (iii) silicon
13. What is meant by the term chemical formulae? Explain with example.
14. An ion M2- contains 10 electrons and 10 neutrons. What is the atomic number and mass number of the element M? Name the element.
15. Given that natural sample of iron has isotopes 54/26Fe and 57/76Fe in the ratio of 5%, 90% and 5% respectively. What will be the average atomic mass of iron (Fe)?
16. Write down the formulae of
(i) Sodium oxide
(ii) aluminium chloride
(iii) Sodium sulphide
17. Write down the names of compounds represented by the following formulae:
(i) AI2 (SO4)3 (ii) CaCl2 (iii) K2SO4 (iv) KNO3
18. Give any two characteristics of isobars.
19. A naturally occurring sample of Lithium contains 7.42% of 6Li and 92.58% of 7Li. The relative atomic mass of 6Li is 6.015 and that of 7Li is 7.016. Calculate the atomic mass of a naturally occurring sample of Lithium.
20. If the number of electrons in an atom is 8 and the number of protons is also 8, then
(i) what is the atomic number of the atom?
(ii) What is the charge of the atom?
Structure of the atom class 9 extra questions (Long answer type Questions)
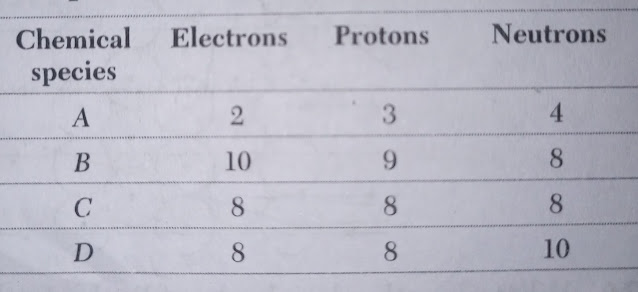 |
| Table |
YOU MIGHT ALSO LIKE
(Join our telegram channel for more material)
Opinion
Thanks for reading our structure of the atom class 9 extra questions and if you solve the all extra questions you will be ready for the chapter test [test is on telegram] hope you will practice well for term 2 examinations. Let us know in comment section about your experience.
FAQ (frequently asked questions)
Question
What is the structure of the atom class 9?
Answer:
The structure of an atom, theoretically consisting of a positively charged nucleus surrounded and neutralized by negatively charged electrons revolving in orbits at varying distances from the nucleus, the constitution of the nucleus and the arrangement of the electrons differing with various chemical elements.
Question
What is an atom? Class 9
Answer:
The smallest tiny particles of matter which can't be divided further is called atom, i.e., an atom is the smallest building block of matter.
Question
What is the Rutherford model of an atom?
Answer:
Ernest Rutherford designed an experiment to know how the electrons are arranged within an atom.
EXPERIMENT
He bombarded thin sheets of gold foil with fast moving α-particles (these are doubly charged helium ions having a mass of 4 u).
GOLD FOIL HI KYU? JYADA AMEER THA KYA?
He selected a gold foil because he wanted a layer as thin as possible. This gold foil was about 1000 atoms thick.
OBSERVATIONS
(i) Most of the fast moving α-particles passed straight through the gold foil.
(ii) Some of the a-particles were deflected by the foil by small angles.
(iii) Very few a-particles (one out of 12000) appeared to rebound.
CONCLUSION
(i) Most of the space inside the atom is empty because most of the α-particles passed through the gold foil without getting deflected.
(ii) Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
(iii) A very small fraction of α-particles deflected by 180° (they rebounded) indicating that all the mass and the positive charge of the atom were concentrated in a very small volume within the atom.
POSTULATES
(i) There is a positively charged, highly dense centre in an atom, called the nucleus. Nearly, the whole mass of the atom resides in the nucleus.
(ii) The electrons revolve around the nucleus in a circular path.
(iii) The size of the nucleus (10-15 m) is very small as compared to the size of the atom (10-10m).{1 LAKH TIMES SMALLER}
Question
What is the JJ Thompson model of an atom?
Answer:
J.J.Thompson was the first one to propose a model of structure of an atom
He said that the positive charge is uniformly distributed in an atom and negative particles are embedded in it
Just like a pudding or a watermelon (red-edible part is positive charge and the seeds are negatively charged particles)
Question
Who discovered neutrons?
Answer:
Discovered by James Chadwick in 1932
Represented by ‘n’
Electrically neutral
Same mass as of a proton
Present in nucleus of an elements except hydrogen
Question
What is the Bohr's model of an atom?
Answer:
POSTULATES
(i) Atom consists of a positively charged nucleus around which electrons revolve in discrete orbits i.e. electrons revolve in permissible orbits and not just in any orbit.
(ii) Starting from the nucleus, energy levels (orbits) are represented by numbers (1, 2, 3, 4 etc.) or by alphabets (K, L, M, N etc.).
(iii) Energy of an electron remains the same as long as it remains in discrete orbit and it does not radiate energy while revolving.
You might also like
- Class 9 gravitation extra questions
- Work and energy class 9 extra questions
- Atoms and molecules class 9 extra questions
- Why do we fall ill class 9 extra questions
- Class 9 gravitation notes
- Atoms and molecules class 9 notes
- Work and energy class 9 notes
- Why do we fall ill class 9 notes
- Class 9 full reduced syllabus of science
- Class 9 term 2 strategy
- Class 9 term 2 mathematics syllabus
- Class 9 term 2 social science syllabus
Conclusion
Hope you like the structure of the atom class 9 notes and structure of the atom class 9 extra questions and thanks for visiting my blog. Let us know in comment section about your experience.



Post a Comment
If you have any doubt or need any study material comment.